[ed note: Michael Jorrin, a medical writer who we have dubbed “Doc Gumshoe” (he’s not a doctor), shares his thoughts with us once or twice a month — what follows is his latest missive, the words and opinions below are his alone.]
I didn’t plan it this way, but the timing of this Doc Gumshoe post couldn’t have been much better. As I was gathering material, news items from the American Diabetes Association’s 74th scientific meeting, which ran from the 13th to the 17th of June, kept popping up.
“Popping up” isn’t a bad way to describe what these news releases were doing. They were mostly modest pops, like back yard Fourth of July fireworks. Some of the news media covering this event did throw around the B word, but if any of the agents discussed at the meeting attain Blockbuster status, it will be because of the magnitude of the problem (and the opportunity!) posed by diabetes, and not because any of the drugs appear to have the capacity to work genuine miracles.
So, what’s the magnitude of the problem?
In Doc Gumshoe’s previous piece about diabetes (“The Why and the What of Diabetes Management”) there was a Tiny Addendum that noted that the Centers for Disease Control had upped their estimate of the number of persons in the US from about 26 million in 2011 to about 29 million today. That’s an increase of a million per year. Note that those figures include both Type 1 and Type 2 diabetes, and that somewhere between 25% and 30% of all diabetes is undiagnosed. Previously the ADA had stated that there were about 1.9 million newly diagnosed cases of diabetes in adults per year. Yes, those numbers are estimates, albeit careful estimates based on the best data.
And here’s another estimate from the CDC: 86 million people in the US have what is labelled “prediabetes,” i.e., insulin resistance or impaired glucose tolerance. The CDC projects that without intervention, about 30% of those persons would have Type 2 diabetes within 5 years.
That would up the total diabetic population of our fair land to somewhere in the neighborhood of 55 million individuals.
My bet is that we won’t reach that figure – that’s a worst-case scenario, and a lot of effort is being lavished on diabetes awareness, screening, healthy living, etc. Just because Mayor Michael Bloomberg’s proposed ban on half-gallon sugary drinks (or whatever size) didn’t go through doesn’t mean that Attention Isn’t Being Paid.
All the same, Doc Gumshoe wouldn’t be a bit surprised if we got to 35 – 40 million cases of frank diabetes by the year 2020.
Here’s something else: United Healthcare projects that the costs of diabetes treatment could reach $500 billion by 2020. I don’t know whether that’s meant to strike terror into the hearts of the citizenry, who would have to fork over that moolah, or to provide an incentive to the pharmaceutical industry to come up with drugs that could snaffle a chunk of that loot. However, as I write these words, the ghost of Miss Truesdell is looking over my shoulder and shaking her head. She doesn’t think that $500 billion number makes sense. The 2012 medical costs totaled $176 billion, and the $69 billion in lost productivity brings the total to $245 billion. I am skeptical about adding lost productivity to the costs of diabetes treatment, but even if we include lost productivity, I wonder whether the cost will really double to $500 billion in 8 years. Anyway, however we figure it, the whole pie is immense and Pharma wants its slice.
Big Pharma steps up to the plate
Despite the recommendation by McKinsey & Company a couple of years ago that big pharmaceutical outfits should ease back on R & D and concentrate their efforts on their “core competency,” that being sales and marketing, Big Pharma is lavishing lots of resources on R & D in the diabetes area. Here’s a quick list (by no means exhaustive) of the clinical trial activity of some of those outfits:
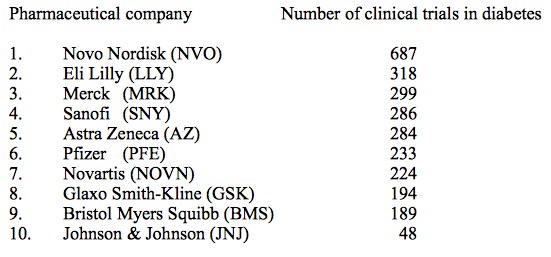
No surprise that Novo Nordisk (NVO) tops the list, since they are the long-established leader in diabetes treatment products, specifically a range of insulins, but also the incretin mimetic Victoza (liraglutide). Further down, we’ll be discussing the benefits vs potential risks of the entire class of drugs that relate to incretins and the rapid insulin response, as well as the duel between Victoza and Eli Lilly’s (LLY) dulaglutide.
And here’s a list of the top 10 diabetes drugs in terms of sales:
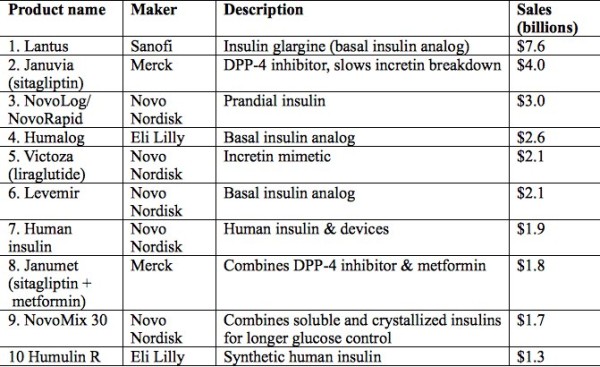
As you see, seven of the ten are insulin products in one of several forms, and five are Novo Nordisk products. This may come as a surprise, since the great majority of persons with diabetes have Type 2 diabetes (T2DM), which is, at least initially, usually treated with non-insulin agents. Several factors contribute to the global dominance of insulin:
- One, eventually almost all persons with T2DM wind up needing supplementary insulin, as their pancreatic beta cells quit functioning after long years of battling against systemic insulin resistance. (We discussed this at greater length in the previous blog.)
- Two, many persons with T2DM, particularly in less-developed regions of the world, have advanced disease at the time of diagnosis, likely beyond the stage where merely increasing the sensitivity of insulin receptors does much good. Exogenous insulin is the quick answer, and mostly does the trick.
Metformin, which is a usual first-line drug for treating T2DM, is also huge seller globally, but it’s relatively cheap. If the sales of generic metformin plus branded formulations such as Glucophage (Bristol Myers Squibb (BMY)) are added, the total dollar amount only hits somewhere between $1.5 billion and $2.0 billion.
However, another trend worthy of our notice: all three of the top sellers that are not insulin products are drugs that target first-phase insulin release through the incretin response. And many of the drugs discussed at the ADA meeting, particularly the ones that attracted the most attention, also address the first-phase insulin response. Why is this?
A bit more about incretins and the first-phase insulin response
In a non-diabetic person, the pancreatic beta cells are secreting insulin all the time, because every cell in our bodies requires insulin to enable the process of converting glucose into energy. Even when we’re sound asleep, our hearts pump, our lungs suck in air, and our brains have dreams or nightmares, as the case may be. We don’t turn off.
This constant insulin release is called the basal insulin response. It accounts for about half the total. The other half, called the first-phase, or sometimes prandial insulin response, is in direct response to food intake. When nutrients of any kind get into the digestive system, a couple of different peptides are released from the gut, mostly from the small intestine, although some are also released from the colon. These peptides are designated GLP-1 and GIP and are generally termed incretins.
What they do is send a message to the beta cells that it’s time to step up insulin secretion, because a load of glucose is being dumped into the bloodstream. In addition, they send a message to the liver that it’s time to stop breaking down glycogen into glucose and releasing that glucose into the bloodstream – it’s okay for the liver to do that when there’s no food in the digestive system, but we don’t need that extra glucose right after a meal.
Incretin release starts quite soon – about 15 minutes after any nutrients pass into the small intestine – and continues for up to 2 hours. The response of the beta cells to this signal is actually more rapid than their response to the presence of glucose in the blood. This has been demonstrated by studies timing insulin response to intravenous glucose injections compared with insulin response to food in the gut. Food in the gut triggers a quicker response.
However, the half-lives of these two peptides are very short – GLP-1 about 2 minutes, and GIP about 5 to 7 minutes. That’s because incretins are broken down by an enzyme labelled DPP-4. The release of incretins and their breakdown by DPP-4 is clearly a homeostatic process – the organism only requires a robust first-phase insulin release during digestion (while glucose is being sent to the bloodstream) but not after.
But in persons with diabetes, the incretin response is not sufficient to trigger the release of enough insulin to bring the blood glucose to normal levels. This may result in that adverse feedback loop, in which high blood glucose brings about increased insulin release, which in turn leads to resistance at the insulin receptors and continued high blood glucose.
Two classes of drugs attempt to remedy this defect. One class consists of the inhibitors of DPP-4 (the breakdown enzyme), thus prolonging the life of the incretins. DPP-4 inhibitors are called “gliptins.” Merck’s (MRK) Januvia (sitagliptin), second on the list of best-selling diabetes drugs, is one of these.
Strong competition for Januvia
One current challenger is Galvus (vildagliptin). Novartis (NVS) is putting the pedal to the metal on this one. Currently 139 clinical trials with vildagliptin are at various stages. Galvus has not received FDA approval, even though Novartis has been knocking at the door since 2007, the reason being that there were hints that, along with other incretin-based drugs, there might be an association with pancreatic cancer. Note, there was no evidence that vildagliptin itself ever led to pancreatic cancer in a patient, and the FDA and the European Medicines Association (EMA) have reviewed huge amounts of evidence regarding sitagliptin (Januvia) and exenatide (Byetta), an incretin mimetic, and found no evidence of increased incidence of pancreatic cancer. The FDA/EMA statement and a summary of the evidence was published in the New England Journal of Medicine in February of this year (N Engl J Med 2014; 370:794).
"reveal" emails? If not,
just click here...
My guess is that as the results are announced of the many safety studies with Galvus, FDA approval for this drug will be forthcoming. Galvus has performed very well in clinical studies; for example, reducing mean HbA1C levels in older patients to essentially normal levels, and also minimizing some of the adverse effects (i.e., hypoglycemia) associated with some other antidiabetic drugs. A competitive disadvantage versus Januvia is that Galvus needs to be taken twice daily, whereas Januvia can be taken once daily. Galvus is likely to be a big boost for Novartis.
The duel between Victoza (liraglutide) and Eli Lilly’s dulaglutide
Both of those drugs are incretin mimetics. Incretin mimetics are not synthetic insulins. Instead, they are entirely different molecules that nonetheless closely mimic the activity of incretins, which is to say that they tell the pancreatic beta cells to release insulin, and they tell also slow the process of converting stored fat (glycogen) to glucose. They do this by activating the GLP-1 receptors on the surface of pancreatic beta cells, but only when there are excessive levels of glucose in the blood. When glucose returns to normal levels, insulin secretion also returns to normal or basal levels – that is, the prandial insulin phase peters out.
Incretin mimetics work in tandem with native incretin secretion from the gut. However, an important difference is that the incretin mimetics are not degraded by DPP-4. While native incretins have a very short half-life, mostly less than 10 minutes, incretin mimetics have a much longer half life; therefore it is feasible for these agents to be dosed as drugs.
Lilly began a series of Phase 3 clinical trials with dulaglutide in 2010, comparing their new agent with a wide range of antidiabetic drugs, including insulin glargine and insulin lispro, metformin, the sulfonylurea glimepride (Amaryl), the DPP-4 inhibitor Januvia (sitagliptin), which is the 2nd-biggest diabetes seller, and the incretin mimetic Byetta (exenatide), as well as in patients with kidney disease, hepatic impairment, patients taking digoxin, and women on oral contraceptives. Trials to date have been uniformly successful; for example, dulaglutide achieved greater reductions in HbA1C than either insulin glargine alone or a combination of insulins glargine and lispro, and also bested Januvia and Byetta.
A greater challenge for Lilly has been the head-to-head comparison with Novo-Nordisk’s Victoza (liraglutide), the top-selling incretin mimetic, and the number 5 seller in the diabetes sweepstakes. In the AWARD-6 trial, dulaglutide lowered HbA1C slightly more than Victoza, by 1.42 percentage points from baseline, compared with 1.36 for Victoza. This means that for many patients who present with HbA1C levels in the neighborhood of 8%, dulaglutide alone could be enough to bring them within acceptable limits, even though it is not likely that this new agent will be approved as monotherapy for newly diagnosed patients.
The very small difference in favor of dulaglutide will not be enough to change the prescribing practices of most physicians. However, there is a major difference. Whereas Victoza is given by injection every day, dulaglutide is given, also by injection, once a week. When dulaglutide receives FDA approval in the US, it will be marketed under the name Trulicity.
Victoza did perform better than dulaglutide/Trulicity in one respect. Patients on Victoza in the AWARD-6 trial lost a bit more weight than those on dulaglutide – 3.6 kg versus 2.9 kg. That result has prompted Novo Nordisk to study Victoza as an obesity drug, perhaps to compensate for their anticipated lost sales when dulaglutide hits the market.
As I have said before, I’m reluctant to use the B-word when talking about a drug’s prospects, but if there’s one where that word might be appropriate, it’s dulaglutide/Trulicity. This couldn’t come at a better time for Lilly, which, as some of you have likely noticed, needs something to wipe the egg off its face after recent flubs with Alzheimer’s and cancer drugs. Dulaglutide will very likely bring substantial rewards for Lilly.
… and now for something new and trendy?
Some industry insiders are calling it a “bionic pancreas.” Here’s how it works. First, for comparison, the standard insulin pump, used by millions of persons with diabetes, mostly Type 1, requires the patient to monitor his or her blood glucose levels by the standard finger-stick method, calculate how much insulin he/she needs at that moment, and instruct the pump to deliver that amount of insulin. It was a huge advance over the old way of getting insulin, which consisted of several daily self-administered subcutaneous injections. The insulin pump is more accurate and can deliver insulin more gradually than the insulin injection. The user can tinker with the way the pump delivers insulin, ranging from a bigger dose initially followed by a trickle, perhaps mimicking the prandial / basal insulin responses, to a more even release throughout the 24 hours. Nonetheless, it’s far from perfect, and some insulin pump users continue to experience potentially dangerous episodes of low blood glucose, and also insufficiently controlled elevated blood glucose.
The so-called bionic pancreas tries to eliminate these issues. The patient has two subcutaneous needle-like devices implanted. One delivers insulin, and monitors blood glucose levels. The other delivers glucagon, the hormone that signals the liver to turn stored glycogen into glucose. The implanted needles also monitor the body fluids for insulin and glucagon respectively, so that these two agents are released into the body as needed. The devices are connected to externally-worn insulin and glucagon pumps.
What controls the whole system is that the information from the implanted devices is transmitted to a smartphone, which does the calculations and instructs the pumps as to how much insulin or glucagon to deliver.
A pilot version of this system was tested by Mass General Hospital in 20 adults and 32 young people. In the pilot version, the patients still used the finger-stick method to monitor their levels, but then input that data to their smartphones. The results strongly favored the experimental system over the insulin pump, lowering blood glucose, in the words of the chief investigator, Steven Russell, MD of Mass General, “to levels that have been shown to dramatically reduce the risk of diabetic complications.”
Perhaps more important, it reduced the incidence of hypoglycemic episodes by 37% in the adults and by 50% in the young people. Although in the long run, complications are what destroy the lives of people with diabetes, on a day-to-day basis what patients and health-care providers most have to watch out for are the low blood glucose episodes – so called “insulin reactions” – which can cause fainting, shock, or coma.
If my instincts are correct, this is a development that will have enormous impact on the lives of persons with diabetes, both in the short and long-term. The likely first market for these devices will be younger persons with Type 1 diabetes. But the Type 2 diabetic who can no longer control the disease with non-insulin agents will also certainly benefit and likely jump aboard..
The next time you see a person looking intently at a device, it might not be a device-addict checking for tweets, but a diabetic controlling his/her condition “on the go!”
As for which medical device outfit will jump on this to make and market the “bionic pancreas,” Doc Gumshoe will keep his ear to the ground.
Salsalate lowers blood glucose in Type 2 diabetes
Salsalate is an NSAID (non-steroidal anti-inflammatory drug) in the salicylate family, related to aspirin, which is used to treat osteoarthritis. There were suggestions that it might be effective in treating diabetes as long ago as the 19th century, but these were based on anecdotal evidence and not seriously pursued once insulin deficiency was identified as the underlying pathology in diabetes. However, it has long been thought that inflammation had a role in the pathogenesis of diabetes, and this has now been confirmed in well-conducted clinical studies.
A shorter study, 14 weeks, evaluating the effectiveness of salsalate was published in 2010. This has now been followed by a 48 week study in 286 subjects with T2DM, comparing salsalate with placebo. HbA1C levels in subjects taking salsalate were 37% lower than in those taking placebo.
And patients in the salsalate group needed fewer additional medications to control their diabetes. In addition to its beneficial effects on blood glucose, salsalate demonstrated some benefit in reducing some markers of cardiovascular disease, although the salsalate cohort experienced small increases in LDL cholesterol.
More than propelling salsalate into the front line of agents used to manage diabetes, it provides clinicians with an additional mechanism of action through which to reduce blood glucose levels. The salsalate research was conducted by the Joslin Diabetes Center, which will continue to investigate this drug’s usefulness in the entire spectrum of cardiovascular disease, including diabetes. However, I think we can anticipate research initiatives into managing diabetes through the anti-inflammatory pathway. A likely avenue would be an oral formulation combining an agent that maximizes native insulin utilization with an anti-inflammatory agent with optimum glucose-lowering effectiveness as well as cardiovascular benefits. This would have multiple advantages. It would help with patient compliance, since the fewer pills the patient has to remember to take, the better. It would address two distinct disease mechanisms – insulin deficiency and inflammation. And it would minimize adverse effects, particularly hypoglycemia. Doc Gumshoe predicts that we won’t have to wait long for such a combination to be developed and marketed, and that it will be a bonanza for the pharma outfit that gets there first.
And one more tiny addendum
Yes, stuff keeps popping up. About a week ago (as I was writing this), a paper was published in the Journal of the American Medical Association (JAMA. 2014 Jun 11;311(22):2288-96) comparing two groups of persons with T2DM who were not attaining acceptable HbA1C levels with metformin alone and required intensification of their drug regimen. This was not a controlled clinical trial, but a large observational study. There were two cohorts, one which added insulin to metformin, and a second which added a sulfonylurea. The main outcome measures were cardiovascular events and all-cause death. While there were no statistical differences in cardiovascular events, all-cause death was about 44% higher in the subjects taking insulin than in those taking a sulfonylurea. The possible causes of this large, significant difference need further investigation, and they could range from baseline differences in the subjects (i.e., the patients who got insulin were sicker to start with), to patients’ problems with managing their insulin dosage, which might be improved with the “bionic pancreas” we discussed earlier. At this point, it’s a red flag. We’ll keep watching.
* * * * * * *
Do please keep the comments coming! They are very important to me, since it lets me know if I’m on the right track sending you stuff that you’re interested in. Right now, I’m planning my next piece – I’m thinking about the placebo effect, which has gotten some attention lately in elevated circles. The question is, is it real? And beyond that, can it be put to use? We’ll see!
While vast amounts of money are being spent toward treating diabetes, spending a small fraction of those amounts on healthful foods would arguably have a greater positive effect. Numerous studies have linked a high glycemic diet (refined starches and sugars) to Type 2 diabetes.
Yes, exactly! For as good a definition of the problem as any, consider:
“…Diabetes UK continues to recommend that diabetic people follow the same balanced diet recommended for the rest of the population…meals can contain some starchy foods such as bread, potatoes, cereals, pasta and rice…”
and
“…Stephen Fyfe, spokesman for the charity in Scotland, says research on the effects of low-carb diets has been limited in scope and duration. “Until the long-term effects prove the effectiveness and safety of low- carbohydrate diets, Diabetes UK does not recommend them.”
Yikes!
More at:
http://forum.lowcarber.org/showthread.php?t=370073
A nice article Michael.
I might elaborate on it somewhat by pointing out that the basis for insulin resistance is still mostly unknown. Just yesterday, an intriguing company called Synthetic Biologics (SYNG) announced that its new agent for killing gut methanogens raises insulin sensitivity by an amazing 50 per cent. Gut methanogens are Archaea. As I have discussed at several junctures in my columns, life on earth comes in three key varieties (in order of age): Archaea, prokaryotes (ie, bacteria), and eukaryotes (plants, animals, fungi). The Archaea are an ancient class of primitive organism, and these often abound in the gut and make methane. How they may influence insulin sensitivity is not yet known. SYNG’s agent doing this is called SYN-010, and thus far the company has not divulged what it is….it is a type of antimicrobial, of course.
Gumshoers may remember SYNG because it is also developing E3, estriol, for MS. It is a very cheap company, and may be one that investors now take notice of.
One increasingly important agent for managing DM-II will be bromocriptine, used to quell GH release by the pituitary. Especially in diabetics on insulin and sulfonylureas, GH elaboration overnight is a vital counterregulatory mechanism, and tends to make diabetics, some of them, begin the day with high glucoses. One of GH’s main actions is that it turns on hepatic glucose output (either by glycogenolysis or gluceoneogenesis). So, some diabetics wake up with a liver cranking out glucose and a pancreas trying to keep up with that by making insulin. Which worsens the whole dreary insulin resistance scenario.
I believe that if people want to invest in diabetes, there is no better way to do that than being in Novo-Nordisk(NVO). It is up 300 per cent since I bought shares, but has farther to go. These is a big pharma company now, that pays a dividend, and yet institutional ownership is a paltry 8 per cent, very likely to rise. When NVO gets Tresiba approved, it will supplant Lantus. It is developing transdermal liraglutide. And it is likely to spin out its medical informatics unit this year as a separate company. This will unlock quite a lot of value for shareowners, who presumably will be granted shares in the new company. I am long NVO, and yes, am talking my own position, of course, but it is a good company to be in.
I genuinely believe that with the advent of Intercept’s FXR ligand, and GenFit’s agent 505, a PPAR-beta and PPAR-delta agonist, that either curing DM-II or delaying its onset by many years, is at hand. The disease is insulin resistance. DM-II is merely endstage insulin resistance….which is why I chide doctors for not treating the insulin-resistant to prevent the onset of DM-II.
http://www.dddmag.com/news/2014/06/eradication-gut-methane-improves-insulin-sensitivity-lipid-profiles
Link to SYNG study.
Dr KSS, did you type that ticker (SYNG) correctly? at yahoo that brings up Synergetics Inc., a development stage company, focused on the development of alternative energy solutions through waste and bio energy management.
I think the symbol should be SYN. Currently at $1.71 with a market cap of $100M
Dr, when you say still mostly unknown might be true but what we do know that by lowering carbohydrates you can definitely improve Insulin Resistance. I think with Ketogenic type diets you can probably cure Type 2 diabetes without any drugs(in most people)
I will take this chance and ask, what is the most important monitoring “tool.” Is it just measuring HbA1C levels every year or morning glucose levels or just do a glucose tolerance test?
What is a optimum morning glucose level for a healthy adult? How much low is better?
If someone has levels between 100-110 for morning glucose, is that already pre-diabetes?
This is also pretty interesting and gaining some movement in terms of supplementation:
http://ajcn.nutrition.org/content/82/3/559.full
Steven: ketogenic diets will most definitely not cure type II diabetes and are quite dangerous. Most people with DM-II have NASH, and ketogenic diets will grossly intensify NASH. I worry people reading here may go and try these diets and truly badly harm themselves. Once insulin resistance is in place, only two things ameliorate it: vigorous aerobic exercise to 70 per cent of target heart rate (220-age) for 20 minutes 3 x week if a doctor says that is OK, and significant weight loss. Diet makes remarkably little difference in insulin resistance.
Any thoughts on Mankind Pharm? Looks like they’ll get approval in mid July for both the Dreamboat delivery system and their inhaled insulin.
What he said above? Anyone on MANNKIND – MNKD ?
I have discussed MannKind in considerable depth and detail on the biotechnology threads after my columns. I would ask that you please go to those sites on Gumshoe and do control-F to find them. I am quite busy at the moment, and rehashing things here when they have been so amply delved into I just cannot do at this instant.
I may have missed it, but was there a stock recommendation in this article?
No. Michael often mentions pharma companies but doesn’t recommend or analyze stocks on the financial side.
I am intrigued by the wonder drug which comes out in Doc Gumshoe’s look at ways to fight diabetes he came up with salsalate (which is not a dance but a relative of aspirin.) My paternal Oma died of diabetes-linked heart disease and my father had adult onset diabetes too, so I have a horse in this race. But despite my huge respect for Dr KSS I disagree with him about Novo Nordisk. If any of the new potential B drugs (the word we cannot say) pan out, it will be the victim. NVO being Danish is part of the Global Investing universe but no in our portfolio.
That’s OK Vivian. I’ll still dance the salsalate with you anytime, even if you don’t like my companies. “All persuasions, no brutality.” NVO did have a problem for many years in that all it did was make new insulins as a way of replacing its old insulins. I think meanwhile they are clearly moving into non-insulin therapeutics for DM-II. Their recombinant factor VII for bleeding in major trauma and in cirrhosis is a killer-app agent I have used many times and love. I bought in 300 per cent ago on the scientific speculation that liraglutide was a great drug and would be approved, and feel it is now very likely to be approved for obesity in non-diabetic patients. They drop 30-50 pounds on it rather straightaway.
I am no doctor but like doc Gumshoe I read a lot of the literature. Of interest to me in the article above is the inflammation connection with diabetes. Cinnamon is often touted for blood sugar control and it is anti-inflammatory like the salsalate mentioned above which helped lower blood sugar. It seems chronic inflammation is a root problem with heart disease, Alzheimer’s, most cancers, and diabetes – gee that is just about most of the old age diseases we die from. And you may remember the fact that statins prevent heart disease possibly more because they reduce inflammation than because they reduce cholesterol (something the body needs). And of course refined sugar causes inflammation (as do many other common foods in the American diet, like corn fed beef instead of grass fed beef). So perhaps for health we should really focus on an anti-inflammatory diet.
One other thing I have read about is the connection between diet drinks and obesity (and then diabetes). I might not get this quite right but it seems the stomach and gut not only sense food present (and releases incretins to get insulin released from the pancreas) but the stomach like your tongue contain taste buds sensitive to sweet. The stomach senses sweet (like glucose) and sends the signal to the pancreas that glucose is on the way to the blood and the cells of the body. But if you have a diet soda mid day (without other food with real calories) that artificial sweetener not only fools your tongue into thinking it tastes like sugar, but it fools your stomach too. The stomach sends the message glucose is coming, the pancreas releases insulin into the blood, but no real calories or glucose is there in the blood. The cells get flooded with insulin but there is no glucose there. If you do that often the cells start to ignore the insulin – insulin resistance. So when that same person eats real sugars or carbs the cells still ignore the insulin, those sugars are not used, so they get stored as fat and worse the person feels lethargic with no energy from burning those sugars and gets less exercise. So the person thinking they are preventing weight gain by drinking diet soda actually gains weight instead. This explanation may not be 100% correct – again I am not a doctor, but this is basically what I have read. Frankly I don’t drink or eat “diet” anything and don’t drink soda – I prefer green tea or water (or beer or scotch!). But if you do drink diet soda perhaps you should eat an apple or a banana or some real food with it for real calories. As they say it is not nice to fool mother nature.
I have the same questions as Steven. I have (fatal) diabetes in my family. My doctor checks my fasting glucose which is always fine. Is this enough testing? BTW I have every risk factor except for being AA, including having had a 9 1/2 lb baby! It seems to me an annual A1C would be warranted. I’ m basically healthy, very few health problems, pretty pristine bloodwork. I don’t want my doc to see me as a hypochondriac! I really don’t want to be THAT patient!
Side effects with liraglutide, Dr KSS?
c193: in my use of it in patients, they generally just don’t do badly with it at all. Its cousin drug exenatide is famed for making people have nausea (which is probably why they lose weight on it….they are eating-averse).
As regards your screening, keep in mind that the disease is insulin resistance, and diabetes is merely endstage insulin resistance. And so, yes, you are wise to ask for screening. Insulin resistance is functionally a high insulin level for a given glucose level, and a high glucose level for a given level of insulin. What I do routinely in patients about whom I am concerned is draw blood for determination of fasting insulin and fasting glucose (after a 12 hour fast). If the product of those two numbers is 700 or above, I deem that patient ipso facto to be insulin resistant and start them on metformin (if they have normal renal function). Metformin use early on can stave off diabetes onset. I have to say that doctors as a profession tend to be starchy and quite reluctant to change their ways no matter how outdated they are, but if your doctor will work with you on this, it could help you. But doing a1c’s is of no value. They will be normal for the first 20 years that you are insulin-resistant. Having had a baby that large, were you gestationally diabetic?
The first glucose was 116, the second (after a sweet breakfast) was normal. The baby is 24 now, just to give you a time frame.
A recent fasting glucose was 92, which raised no concerns with my PCP. Your comments here match my own reading, and I believe I will pursue further testing.
Thanks so much for your kind concern!!
I guess what puzzles me is why fasting insulin and fasting glucose aren’t routinely tested together, at least when certain risk factors are present. Doctors seem to rely on glucose alone.
What is “the B word”? Is it too nasty dirty for our sensitive ears? Or is it used by those who can’t cope with actual syllables?
Blockbuster
I plan on keeping my little holdings in NVO for a good, long time, in spite of the fact that they pay their annual dividend only once a year. When I was very young, a physician I used to know told me that each and every one of us would eventually get type-2 diabetes if something else had not killed us first.
Joan: just wait’ll they get Tresiba approved, and get Victoza approved for non-diabetic obesity. THEN this one will be on fire and institutions will pile in. It’ll then have 6 of the 10 best selling diabetes drugs.
I agree with Dr. KSS and Joan from Houston: Novo Nordisk is a great company that possibly is a good play on the long run. But another danish company that might interest you is Zealand Pharma. Their product Lyxumia is approved in Europe and Japan and is under a license-agreement with Sanofi. A once-daily single injection of Lyxumia and Lantus is in Phase lll development which might be a huge succes when you consider Lantus’ market share. This is of course a lot more speculative play than Novo Nordisk but could turn out very profitably in a year or two.
Disclaimer: I own shares in Zealand Pharma.
That’s an interesting company ED. That agent is lixisenatide, a GLP-1 RA like exenatide and liraglutide. I am not sure on its PK particulars, and I tend to wish they would not bundle it with Lantus, as to my mind the whole object of using GLP-1 RA’s is avoidance of insulin, which can be quite harmful as it fosters growth of arterial disease. Even so, this one looks a little undervalued here, and promising. A good investment, it seems. One wonders if their GLP-1 is differentiated enough that Novo might just acquire them. The ischaemia reperfusion peptide looks most interesting to me…in actual fact, that agent could work quite nicely in liver transplant patients, where an organ is out of the body, and then is suddenly hooked up to a new hepatic artery. I wonder if they have thought of that. Interesting company.
New developments keep emerging. A small outfit called MicroBiome Therapeutics has done some some animal studies suggesting that the way metformin works to improve glucose control is to induce changes in the composition of the microbial population of the GI tract – called the microbiome. One particular bacterial strain appears to stimulate the production of T-cells that reduce the prevalence of a type of cytokines that promote inflammation and are associated with glucose intolerance. Metformin, they allege, encourages these particular beneficial bacteria and that accocunts for part of metformin’s effectiveness in diabetes. Now MicroBiome has announced the results of a small study with their first agent, a so-called microbiome modulator, designated NM504, no name yet. In this study, subjects that took NM504 had significantly lower blood glucose levels after drinking a sugary drink than did those who took the placebo. At this point, the study is no more than a proof of concept – modulating the population of the microbial inhabitants of the gut could have a beneficial effect on managing diabetes. But it does support the link between inflammation and diabetes – or as Dr KSS would have it, the progression of insulin resistance – as does the very preliminary evidence that the anti-inflammatory salsalate is effective in managing diabetes.
I concur with Dr KSS that insulin resistance should be treated as soon as it is detected. The problem is that for way too many of us in this wealthy nation, diabetes is not diagnosed until it has progressed to the point where serious and irreversible complications have already begun. People with insulin resistance seldom have any symptoms whatever, and are apt to be reluctant to accept treatment that requires serious changes in diet.
I need to add a personal note: my father was diagnosed with diabetes at age 46, back when the only treatment was insulin. He was thoroughly compliant to treatment, kept to a strict diet, was trim and active, experienced regular episodes of hypoglycemia, and died at the age of 63 of a heart attack, as many diabetics do. If he had had the currenty- available treatment options at his disposal, who’s to tell how long he would have lived. Huge progress has been made, and certainly, more is on the way.
Michael, what is your opinion of this question
“Is it just measuring HbA1C levels every year or morning glucose levels or just do a glucose tolerance test?
What is a optimum morning glucose level for a healthy adult? How much low is better?
If someone has levels between 100-110 for morning glucose, is that already pre-diabetes?”
I say none of the above. None of those gets at the crux, which is insulin resistance. The only sensible test is testing for insulin resistance, as outlined by me in a post above.
Now I see. Thank you.
I think an obvious issue is that salsalate cannot be a reasonable adjunctive rx for DM-II because it will only heighten kidney injury. NM504 is a plant derived material, probably fiber, that appears to function as a prebiotic for Akkermansia muciniphilia, which was only discovered about a decade ago: a Gram-negative anaerobe. I have to say that if MicroBiome were serious about this strategy, they would simply manufacture Akkermansia as a probiotic in capsule form, and dispense with all the mystery surrounding their drug, which again is probably just fiber. It’s as if they want to add chronicity to the problem, instead of merely fixing it.
Keith: Sorry, it is SYN. My goof.
Despite several searches, I am still not sure the nature of the archaea-killing drug they have.
Ok. Thanks. I’ve found it now. Looks interesting.
Way out on the edge of irrelevance here (but maybe not in the future as more of the involvement of the diverse, variable human microbiome in the gut and elsewhere in the body in normal human metabolism and in disease becomes known), a bit of clarification concerning the currently recognized three major domains of life is as follows: Both Bacteria and Archaea are prokaryotes (cells without a nucleus, etc.), with Bacteria being the older (coming first in time) on the evolutionary tree. The domain Eukarya covers all eukaryotes (cells contain a nucleus [or two or more nuclei in a relatively few taxonomic lines], etc.) Cell surfaces, where cells first experience and deal with anything (such as a drug) introduced to affect cells, vary within domains and, significantly, between domains.
Carl: the problem with this is that when one labels archaea and bacteria as both being prokaryotes, this implies that they are more closely related to each other than either are to eukaryotes. And that is not what the most current data suggest. There was a primitive putative ancestor, chemoautotrophs, and something broke away from that lineage to form bacteria. The lineage went on for quite some time, and then eukaryotes were a divergent line from it, such that the old archaea seem to be the oldest life form in the fossil record, but also oddly closer, based on RNA operons and HSP70, to eukaryotes than to bacteria. An acquaintance has done much work in this area, and is responsible for revising the model and placing archaea closer to eukaryotes.
I visited my eye doctor and were told to get examination for possible diabetes 2. It were not quite critical but acted as a reminder of cutting down on sugar . My weight were 83kg at that time. Within a year I cut my weight down to 60kg with the help of a dietitist. I eat a lot less and soda is replaced with tapwater. I do own Novo, they are working on a pill solution and they claim they are getting close.
I find the articles by Doc Gumshoe most interesting and in a language that I can understand. The ladies in my family have been dessimated by pancreatic cancer and any information on a diagnostic testing system would be much appreciated. Unfortunately and currently, once it is discovered it is too late to remedy. Age also does not seem to be a factor.
you need to look at the glycemic load of the food and not the glycemic index
I guarantee if you cut out rice, potato, white bread, pasta you will drop a significant amount of weight and your blood sugar will also go down
Also for inflammation try turmeric
Cinnamon also helps reduce sugar
also get off you bum and do some exercise
I agree with Dr KSS that a blood test for insulin resistance would be the optimum diagnostic tool to detect Type 2 diabetes in the earliest stages. But I think the chances of such a test becoming routine for all persons who might be considered to be “at risk” is slim. There are probably 100 million such persons in the US alone, since just about anyone who is getting up there in the BMI numbers could be considered at risk.
No, salsalate will not become a standard anti-diabetic drug. But an oral anti-inflammatory agent, perhaps compounded with one of the druts that optimizes insulin utlilization, might have the effect of employing two different anti-diabetic strategies while keeping adverse effects to the minimum.
And, yes, Akkermansia is the beneficial bug in the gut. Probably NM504 just tinkers wth the different microbial populations in favor of the good bug. We’ll see what comes down the pike.
To Mike Nonya: we have really discussed MannKind almost to death on other threads. You might want to control-F those. I have gone into enormous detail about it.